With statewide and national attention focused on it, Colorado’s Prescription Drug Affordability Review Board declined its first opportunity Friday to move toward capping prices on a medication, declaring that a “miraculous” cystic fibrosis drug is not unaffordable.
The unanimous vote, which came after significant outcry from cystic fibrosis patients and pharmaceutical manufacturers that the board even considered regulating the prescription’s cost as part of its oversight duties, brought with it relief from the Colorado Bioscience Association. Patient-advocacy groups like the Colorado Consumer Health Initiative, meanwhile, said that while they supported the decision, the process showed both why the work of the controversial PDAB is necessary and how more transparency continues to be needed from drug makers.
Legislators created the PDAB in 2021 and strengthened its powers earlier this year, believing the board could be a powerful tool against the escalating prices of prescription drugs. It has the ability to select drugs for affordability review — having picked the first five such medications in August — and then, if it deems them unaffordable, to place an upper payment limit on what the drugs can be sold for in Colorado.
The debate over the drug Trikafta
The first of the five chosen drugs that it reviewed was Trikafta, a four-year-old medication from Vertex Pharmaceuticals that’s been proven to reduce hospitalization of cystic fibrosis patients by 74% and to cut the risk of death from the rare genetic disorder by 72%. A review done by PDAB staff found that Colorado patients and payers — a user number estimated to be between 450 and 700 state residents — spent $108.1 million on the drug in 2022, with the average cost per person reaching $234,439, including $8,907 in out-of-pocket payments.
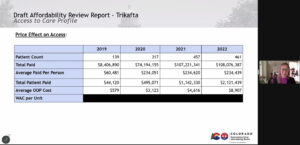
Lila Cummings, Colorado prescription drug affordability director, explains the drug’s costs in a slide presented Friday during the PDAB meeting.
Several PDAB members expressed concern that Trikafta’s out-of-pocket cost nearly doubled from 2021 to 2022, rising from $4,616. Several noted also, however, that Vertex offers an extensive financial assistance program to patients to keep down the cost of the drugs, even as critics argued that the manufacturer had reduced assistance in the past year.
In the end, though, the five PDAB members seemed particularly swayed by the fact that are no similarly effective therapeutic alternatives to Trikafta and by the complete lack of Trikafta patients who complained to the board that pricing for the drug was out of their reach. Cystic fibrosis patients instead expressed fear that if Colorado were to take a first-in-the-nation step of setting an upper payment limit, the drug’s manufacturer would decline to sell it in the state — an argument that is likely to return with other drugs considered in the coming months.
“This is an extraordinarily expensive drug … But it is also an extremely beneficial drug, a miraculous drug that is making a big difference in Coloradans’ lives,” said Dr. Gail Mizner, PDAB chairwoman. “At this point, it does not seem unaffordable. And I would be willing to look at it again if that changes.”
Bigger-picture impacts from PDAB decisions
While six other states have authorized creation of boards like the PDAB, the Colorado body was the first to advance to the stage of casting a vote on the affordability of a drug. Had members determined that Trikafta was unaffordable, they would have met again sometime in the next two weeks to vote on whether they wanted to place an upper payment limit on the drug.
CBSA officials warned Gov. Jared Polis and legislative leaders that the mere existence of the board has begun to affect investment into the bioscience industry, where funding is desperately needed as drugs can spend a decade in research and development at a multibillion-dollar cost. Funders shy away from risk, and the possibility that drug makers may not be able to recoup full investment into their products has created questions on whether there are safer places to put big money, CBSA President/CEO Elyse Blazevich previously told The Sum & Substance.

Amy Goodman is vice president and counsel for policy and advocacy for the Colorado BioScience Association.
“Government price caps are the wrong solution for patients, who are worried about the serious, unintended consequences of price caps on medications,” said Amy Goodman, CSBA vice president and counsel for policy and advocacy, in lauding Friday’s decision. “Government price controls won’t reduce patient out-of-pocket costs. They limit choice and could reduce investments in new medicines.”
Health-care advocates say continued drug review needed
Bethany Pray, interim director of the Colorado Center on Law and Policy, argued that even as insurers cover the vast amount of the cost of Trikafta, the lowest-income patients may not have insurance or may not be able to afford any copays, leaving the drug unattainable to them.
Meanwhile, Hope Stonner, the CCHI policy manager who’d argued that even so-called orphan drugs without therapeutic alternatives need to have their affordability examined, said afterward that she did not take issue with the board’s ruling. But she and several Denver-area physicians noted that the affordability review still was able to show how much profit Vertex was making off Trikafta and how little transparency there was to its financial-assistance program, and they said the work of the PDAB must continue.
“Consumers across Colorado shouldn’t have to shoulder the burden of higher premiums due to greedy corporations,” said Dr. Yolanda Bogaert, a Wheat Ridge nephrologist and member of the Committee to Protect Health Care. “We truly look forward to the board reviewing more drugs and hopefully setting limits on the ones that are unaffordable.”
Next for the PDAB
The PDAB currently is reviewing two other medications — autoimmune-disease drug Enbrel and HIV treatment Genvoya – and is expected to vote on their affordability in February. Then, it’s next scheduled to put under its regulatory microscope Cosentyx and Stelara, both drugs for skin-disease psoriasis and psoriatic arthritis.
But for the first drug it considered, at least, the board showed considerable restraint, acknowledging the unique place Trikafta has in the medical ecosystem and the payment offsets that are being made by manufacturers and insurers to help patients. And members clearly seemed to nod to patients whose concern was about access more than price, adding complexities to PDAB’s decision-making process now and in the future.
“The most vulnerable in our society are those who are depending on you to keep access to this drug,” Jen Reinhardt, mother of a cystic fibrosis patient, said during public comments just before the board took its vote. “The prospect of losing access to this drug really keeps us up at night.”
