Colorado’s precedent-setting Prescription Drug Affordability Review Board has declared a second medication to be unaffordable, setting it up for a possible upper price limit after the board’s decision to consider such a limit on another drug was met with a lawsuit.
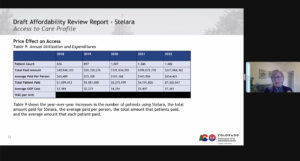
The latest drug to earn that label from the appointed board is Stelara, a medication that treats anti-inflammatory diseases affecting joints and bowels. Stelara, made by Johnson & Johnson, is increasingly popular, with the number of Coloradans using it having risen 250% since 2018, but it also is increasingly expensive, with the average out-of-pocket cost to state residents rising from $2,584 in 2018 to $7,365 in 2022.
PDAB members acknowledged during several hours of deliberation Friday that Stelara’s effectiveness is leading to its increased usage, even as it has about a half-dozen competitors in the market that are, by some comparisons, less expensive. Board chairwoman Dr. Gail Mizner said that while there are other medications that treat arthritis, as Stelara does, there are fewer options for treating inflammatory bowel diseases such as Chron’s Disease and ulcerative colitis.
“If it wasn’t working, there would be other drugs you could go with,” said Dr. Amy Gutierrez, another PDAB member.
A debate of costs versus benefits of prescription drug
But the cost of the medication, which is covered by seven of the 10 health-insurance carriers regulated by the Colorado Division of Insurance, averages $154,401 annually — $7,365 in out-of-pocket costs and the rest covered by health plans. That cost is high enough that its impacts go beyond the 1,606 Coloradans who took the drug in 2022 to affect the overall premiums that small-group and individual insurers charge all users, Gutierrez said.
PDAB members acknowledged that the Janssen Pharmaceutical Companies of Johnson & Johnson offers significant help through its patient assistance program to bring down costs for patients, but board members said they don’t feel comfortable relying on such help. Manufacturers could curtail or end such a program, and some patients don’t qualify for that aid, several argued while noting that the average out-of-pocket costs of the drug rose 87% from 2019 to 2020 and then jumped another 36% from 2021 to 2022.
Lila Cummings, deputy commissioner of health policy for the Colorado Department of Regulatory Agencies, explains a review of the drug Stelara to the Colorado Prescription Drug Affordability Review Board during a Zoom meeting on Friday.
“What if medications were at the price point where we wouldn’t have to have so many people on patient assistance programs in order to afford it in the first place?” said Cathy Harshbarger, a PDAB member and former hospital executive. “Why is it the pharmaceutical companies don’t have the ability to lower their prices to make it affordable?”
PDAB members then voted 5-0 to declare Stelara to be unaffordable. They will vote on Friday whether to consider placing an upper payment limit on what pharmacies could pay for the drug, kicking off what could be a months-long process to determine that price cap should they go in that direction.
Board vote brings divergent reactions
Stelara become the fourth drug to undergo affordability review by PDAB members, who previously declared cystic fibrosis treatment Trikafta and HIV medication Genvoya not to be unaffordable and autoimmune-disease drug Enbrel to be unaffordable. One month after the board began the process to determine an upper payment limit for Enbrel, however, manufacturer Amgen sued the state over the constitutionality of a board that was the first in the U.S. to begin a price-capping process, according to the Denver Post.
Friday’s decision brought similar praise as the Enbrel decision from groups that supported the 2021 creation of the PDAB and similar condemnation from observers who argue that the whole process will backfire on the cost-saving aims of the state.
The Colorado Consumer Health Initiative said in a statement that it supported the decision, calling the existence of manufacturer-sponsored patient-assistance programs “often a fair indicator of unaffordability.” The rising numbers of Coloradans using the drug don’t include the number who have been priced out of using the treatment, and requiring manufacturers to price the medication within reach of patients is necessary for it to have a positive effect, it said.
“Patients need to be able to afford their vital medications,” CCHI communications manager Priya Telang said in a news release. “Life-changing medications truly have no purpose if their patients can’t take them.”
Could vote keep prescription drugs out of state?
But Lisa Pulver, strategic market director for regional accounts for Johnson & Johnson, said that consideration of the payment assistance program — which allows two-thirds of Colorado patients to pay $50 or less for Stelara — clearly marks the drug as affordable. And Cliff Dew, who works with independent pharmacies throughout the U.S., warned that the decision could prompt the drug manufacturer to discontinue selling Stelara in Colorado while it remains available to residents in other states.
“As the PDAB decides whether to set an upper payment limit on a drug it has deemed unaffordable, Colorado BioScience Association continues to stress that government-imposed price caps are the wrong solution for patients,” CBSA vice president Amy Goodman added in a statement to The Sum & Substance. “Government price controls won’t lower costs for patients and risk serious, unintended consequences, including limiting patient and prescriber choice and reducing investments in new medicines.”
Just because the board voted to declare Stelara unaffordable doesn’t mean it will move forward on a cap, but the crucial vote was the first step in the process. And it’s likely that legislators will watch the upcoming discussions closely.
Colorado legislators passed a bipartisan bill this session that requires the PDAB to take input earlier in the process from makers and users of “orphan drugs” — those medications like Trikafta that treat diseases affecting 200,000 or fewer Americans. That came after a range of legislators, including some who supported creation and expansion of the PDAB’s abilities, expressed concern that considering declaring Trikafta unaffordable and placing a possible price cap could bar state residents from being able to get it.
